Describe the Size of an Atom
An atom is the size of a plastic building block. The larger the size of atom lesser is the ionization energy.

See The Electron Configuration Diagrams For Atoms Of The Elements Potassium Atom Electron Configuration Atom Diagram
The size of these atoms is said to have an order.
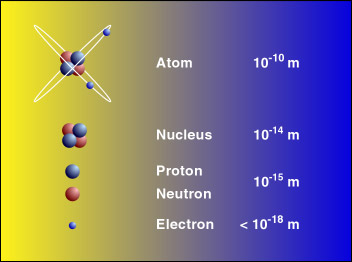
. Size of atoms Atoms have a radius of about 01 nm nanometres or 00000000001 metres 1 10-10 m. R R R0A1 3 R 0 A 1 3. Describe on Size of Atom.
One possible answer to this question is an exact measurement. An atom is small but can be seen with just our eyes. Structure of an atom.
This means that the size of the nucleus is smaller than 410 -14 m. Get an answer for describe how the size changes when an atom forms a cation and when an atom forms an anion and find homework help for other Science questions at eNotes. An atom is the smallest unit of ordinary matter that forms a chemical element.
Where R 0 1210 -15 m. We all know atoms are very small but how small exactly. What is the approximate size of an atom.
The building blocks of matter Do atoms in oxygen double in size. The best way they are described is. The Electrons negative charge protons positive charge and the neutrons no charge.
The nucleus has a very small size but a very high density compared to the overall size of the atom. The Hydrogen atom has a distance between the nucleus and the electron of. The protons and neutrons make up the nucleus the center of the atom and the electrons fly around the.
The volume of the atom is 2 x 1013 times larger than its nucleus. The size of an atom can get defined by the situation. A good comparison of the nucleus to the atom is just like a pea in the midst of a track.
The diameters are slightly different but are all about 10-10 m. Where is it found in the atom. For example the electron arrangement of a chlorine atom is 287.
A For spectrum you want the radius of each subshell. How big is the nucleus in comparison to the entire atom. Every solid liquid gas and plasma is composed of neutral or ionized atoms.
B For bonding you want the distanceradius nucleus to nucleus. This is due to the fact that electrons are tightly held in smaller atoms whereas in large atoms electrons are held quite loose ie lesser energy is required for removal of electrons from. Explain the relative size of the nucleus in relation to the size of the entire atom.
Structure of an Atom The model of an electric carbon which consists of 6 protons 6 electrons and in most cases 6 neutrons has electrons in simple rings or shells surrounding the nucleus. The nucleus of an atom is less than frac 1. Therefore the radius of an atom is more than 10000 times the radius of its nucleus 110 fm and less than 11000 of the wavelength of visible light 400700 nm.
D It is the size of an apple or a large piece of fruit. The sizes of the nuclei of various elements have been accurately measured after conducting many more iterations of the experiment. B It is so small it cannot be seen without magnification.
Size of an atom. An atom is larger than a sheet of aluminum foil. Describe the size and scale of an atom.
Daltons model of atom described the atom as the smallest indivisible particle of matter. Having done this a formula to measure the size of the nucleus was determined. How could you describe the size of an atom.
Where is it found in the atom. Here are some typical atomic diameters. Scientists define this amount.
Atoms are the smallest of anything. But the size is so tiny that its difficult to realize based on the. C It is the size of a grain of sand and can be seen with our eyes.
The ionization energy decreases with the increasing size of atom. He was awarded the nobel prize in physics in 1906 for his work on the discovery of electrons. Atomic Mass Protons and neutrons have approximately the same mass about 167 10 -24 grams.
Size of a nucleus. Nothing smaller than the atom. Compare the relative size and relative density of a nucleus to its atom.
The atom itself is about 50000 times larger than the nucleus a proton. There are 7 electrons in the outermost occupied shell of the chlorine atom. Atoms vary by size but consider a hydrogen atom.
This means it is about 10-5 or 1100000 of the size of the whole atom. This means that if the nucleus were a typical pea then the atom would be about the size of a baseball field. Dynamic Science I The tendency of an atom to attract electrons is.
Every atom includes electrons and a nucleus where protons and neutrons reside. An atom is the smallest particle that can create a chemical composition. As you can see the nucleus is significantly smaller than the atom which is quite superb if you ask me.
Bohr radius - Wikipedia. Atoms are extremely small typically around 100 picometers across. What is the volume of the atom what is the density of the nucleus etc.
Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm trillionths of a meter or between 03 and 3 ångströms. The nucleus of an atom is about 10-15 m in size. Therefore the number of valence electrons in a chlorine atom is 7.
A good comparison of the nucleus to the atom is like a pea in the middle of a racetrack. The atom is made of three different parts. The size of the nucleus of an atom is about 10 raise to power 15 meter which basically proposes that it is about 10 raise to power -5 or 1100000 we can say of the size of the entire atom.
Yes atoms do double in. The number of valence electrons in an atom can be determined from its electron arrangement. A It is so large it is bigger than a tree in the forest.
Atoms are made up of protons and neutrons located within the nucleus with electrons in orbitals surrounding the nucleus. An atom is tiny and cannot be seen without magnification. Briefly describe the structure and size of an atom.

The Size Of Atom Decreases Across A Period From Left To Right Description From Streamscience Blogspot Ionization Energy Chemistry Chemistry Periodic Table

Diagram Of An Oxygen Atom Atom Project Atom Model Project School Science Projects

Eufisica The Evolution Concept Of The Atom Evolution Atom Science Nature

Periodic Property Size Of The Atom Atomic Radius Science Chemistry Atom Thermodynamics

2 5 The Structure Of The Atom Chemistry Libretexts

High School Chemistry Atomic Size Wikibooks Open Books For An Open World

The Atomic Radius Is In Shown In The Middle Of This Chart At 143 In Group 3a Teaching Chemistry Chemistry Lessons Chemistry Worksheets

Atomic Size Introduction To Chemistry

Models Of The Atom Timeline Youtube Atomic Theory Chemistry Chemistry Revision

Structure Of An Atom Chemistry Classroom Chemistry Lessons Organic Chemistry Study

Boron By Carlos Clarivan Atomic Structure Boron Chemistry Worksheets
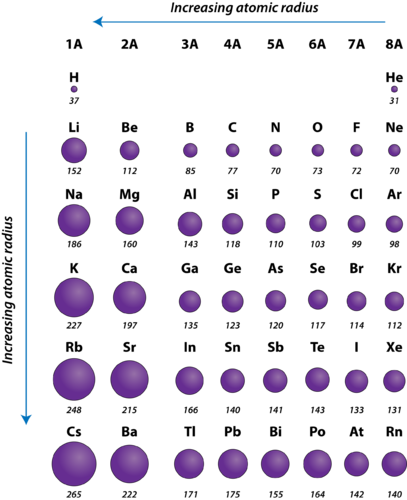
Periodic Trends Atomic Radius Chemistry For Non Majors
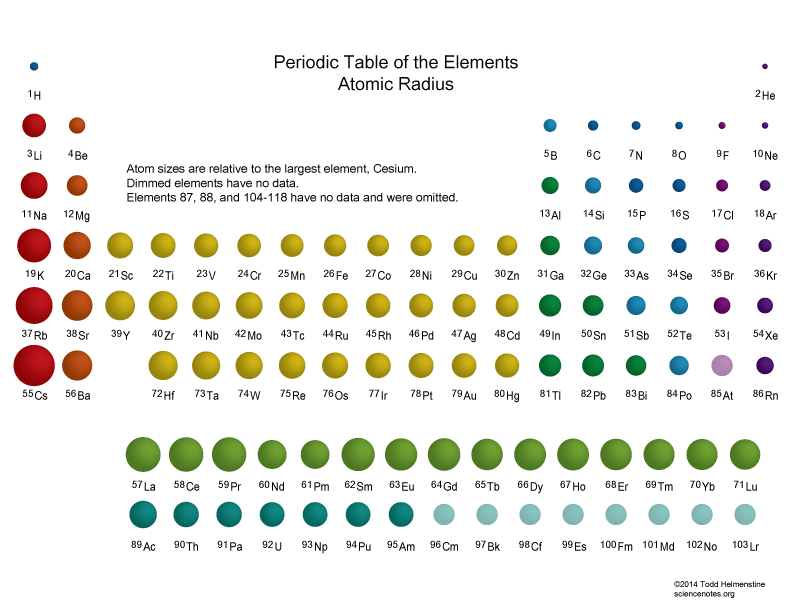
Which Are The Smallest And Largest Atoms Socratic

Atomic Size Atomic Radius Definition Variation In Periodic Table With Videos Of Atomic Radius

What Is A Molecule Definition And Examples Molecules Chemical Bond Hydrogen Bond




Comments
Post a Comment